Specimens
Guidance on specimen collection, packaging and transport, and rejection criteria.
A properly collected specimen is critical to quality test results. Ensure that:
The correct specimen type is collected.
The correct amount is collected.
The specimen is collected in the right container with any necessary additives.
The specimens are collected following safe working practices.
Ensure that there is no contamination from external sources when collecting microbiology and virology samples.
Clean surgical instruments and surgical trays must be used when collecting Histopathology samples.
The container is securely sealed and labelled.
Specimen collection
ThinPrep® PAP Test Cervex Brush Protocol
PREPARE ALL EQUIPMENT BEFORE STARTING THE PROCEDURE
Note expiry date on sample collection vial. Do not use expired vials.
Ensure the entire plastic seal is removed from the lid of the vial and discarded.
Complete patient details on both the request form and the vial. Specimens may be returned or discarded if details are missing from the vial.
Remove the lid from the vial before taking the sample.
Use of lubricant is not recommended.
Do
If excessive mucus is present, this should be gently removed before sampling.
Use either the Cervex Brush (broom-like device) on its own or a Plastic spatula and endocervical brush combination.
The Cervex Brush should be rotated 5 times in a clockwise direction. The Plastic spatula should be rotated through 360 degrees and the endocervical brush rotated through one quarter to one half turn.
Immediately rinse the collected material into the vial.
Replace the lid and tighten so that the black torque line on the cap passes the black torque line on the vial to avoid leakage.
Keep the unlabelled portion of the sample vial free of labels so that the contents can be seen.
If barcoded labels are used these must be applied horizontally around the vial.
Samples should be sent to the laboratory without delay.
Don't
DO NOT leave the head of the Cervex Brush in the vial.
DO NOT routinely clean the cervix or take a cervical swab before taking a cervical sample.
An endocervical brush should never be used in isolation.
DO NOT under any circumstances use a wooden spatula.
DO NOT leave the collection device sitting in the vial whilst dealing with the patient.
DO NOT over-tighten the lid on the vial.
DO NOT place multiple labels on the outside of the vial.
DO NOT apply barcoded labels vertically on the vial.
DO NOT use expired vials.
DO NOT delay the sending of vials to the laboratory. The sample needs to be processed within 3 weeks of collection.
DO NOT use excessive lubricant – please avoid if possible.
Step:1. Record
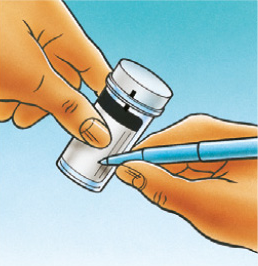 ...the patient’s 3 identifiers to include date of birth on the vial.
...the patient’s 3 identifiers to include date of birth on the vial.
.....the patient information and medical history on the cytology requisition form.
Step:2. Obtain
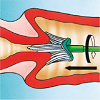 …an adequate sample from the cervix using a Cervex Brush (broom-like device). Insert the central bristles of the brush into the endocervical canal deep enough to allow the shorter bristles to fully contact the ectocervix. Push gently and rotate the brush in a clockwise direction five times.
…an adequate sample from the cervix using a Cervex Brush (broom-like device). Insert the central bristles of the brush into the endocervical canal deep enough to allow the shorter bristles to fully contact the ectocervix. Push gently and rotate the brush in a clockwise direction five times.
Step:3. Rinse
 …the Cervex Brush immediately into the PreservCyt Solution vial by pushing it into the bottom of the vial 10 times, forcing the bristles apart. As a final step, swirl the brush vigorously to further release material. Visually inspect the Cervex Brush to ensure that no material remains attached. Discard the brush.
…the Cervex Brush immediately into the PreservCyt Solution vial by pushing it into the bottom of the vial 10 times, forcing the bristles apart. As a final step, swirl the brush vigorously to further release material. Visually inspect the Cervex Brush to ensure that no material remains attached. Discard the brush.
Do not leave the head of the Cervex Brush in the vial. Check the vial is in date before use.
Step:4. Tighten
 …the cap so that the black torque line on the cap passes the black torque line on the vial. Do not over-tighten.
…the cap so that the black torque line on the cap passes the black torque line on the vial. Do not over-tighten.
Step:5. Place
 …the vial and request form in a specimen bag for transportation to TDL.
…the vial and request form in a specimen bag for transportation to TDL.
Cytology (Non-Gynaecological)
TDL’s Cytology team carries out microscopical examination of cells or crystals on a range of non-gynaecological body fluids, including urine, sputum and CSF.
Urines
To prevent cell degeneration, it is advisable to collect urine samples in a sample pot containing preservative (available from TDL Supplies). Use of preservative will ensure that the cellular material is preserved for up to 48 hours.
Ideally, 10ml (excluding preservative) from a freshly fully voided urine (when the bladder is emptied) mid-morning sample should be submitted for cytological assessment. If microbiology or chemistry investigations are also required, please submit separate urine samples and mark the vials accordingly.
A mid-stream urine sample is NOT recommended for cytological assessment, as it could lead to a low cellular yield. If a delay of greater than 24 hours in reaching the laboratory is anticipated, samples should be refrigerated at 4°C.
Sputum
Sputum should be collected on at least three occasions if underlying lung carcinoma is suspected.
A single sputum is sufficient for microbiological assessment. Sputum should be sent to the laboratory immediately following production, or stored in a universal container containing cytolyt cell fixative if there is likely to be a delay. Please note that this is only acceptable if sputum is only for Cytology. Microbiology cannot be performed on fixed material.
Early morning sputum is ideal, but contamination with food, toothpaste and tobacco should be avoided.
Fluids
All available material should be submitted in a sterile container without fixative as quickly as possible. If any delay is anticipated, the material should be submitted in cytolyt fixative.
Cerebrospinal fluid (CSF)
Ideally, CSF should be submitted fresh or as an air dried cytospin slide, unstained and in a plastic transport slide box. A minimum of 3ml should be submitted, either in fresh form or spun on multiple slides for cytopathologists’ review and opinion.
Sample requirements and request procedures
Transport to the laboratory, required patient details, consent forms, and requests for additional tests.
Sample transport arrangements
All specimens should be kept at room temperature and despatched to the laboratory as soon as possible, by TDL/international courier, first class post, guaranteed next day delivery or a reliable alternative.
If a delay in sending the sample is unavoidable, please refrigerate overnight – DO NOT FREEZE. For NIPT sample stability see here for more information, do not refrigerate or freeze NIPT bloods.
Specimens must not be allowed to come in contact with request forms, but should be kept separate by using dual – pocketed plastic bags. Specimens for inland postage must be packed in a rigid crush-proof container according to current Post Office guidelines. IATA guidelines should be followed for international transport (Advice is available from the laboratory).
Labelling of high risk samples
Please note that it is the responsibility of the referring clinician to ensure that high-risk samples are clearly identified to reduce the risk of infection to staff and others.
Patient details on request forms and samples
Request and consent forms are available on this website.
To avoid unnecessary time spent in obtaining details, please provide the following information:
Information for request forms:
Surname, forename (not initials), date of birth and biological sex of patient for postnatal referrals
Full name (not initials) and location of referring clinician
Full address of clinician to whom the result should be sent
Legible clinical summary, including details of any relevant family history
Address for billing – Doctor, patient or other
Gestation on prenatal samples
Hospital or reference number
Test required
Essential information on sample container label:
Patient’s surname and forename (not initials)
Date of birth
Hospital number or reference number
Consent forms
Consent forms are available for genetic testing. As genetic testing may have implications for other family members and is regarded as personal data, it is recommended that written consent is obtained wherever possible. In cases with predictive testing for severe disorders, as indicated in the laboratory guide, it is essential that patients should also be offered formal genetic counselling. It is the responsibility of the referring clinician to obtain appropriate consent from the patient.
Unlabelled samples
Unlabelled samples will ONLY be processed if the individual who took the sample can confirm the sample is from the patient in question. In the absence of this assurance, the sample will be discarded and a repeat required.
Requesting additional tests
Any further tests not requested at the time of sample receipt must be requested within:
1 week for tests requiring prenatal culture or cultured cells
2 weeks for DNA testing
2 weeks for cell culture testing
3 months for FISH testing
Samples can be stored for longer periods if specifically requested at the time of sample receipt.
Medical microbiology
TDL's Microbiology service is a broad- ranging, clinically-led pathology service. In addition to routine microbiology diagnostics, the laboratory includes reference and developmental clinical services, with expertise in all areas of conventional and molecular microbiology.
Microbiology tests
Test codes, sample requirements, and turn-around times details are avaliable in the AIA LMS platform.
Urine culture processing and results
Urine culture testing is performed using manual methods.
All urine culture testing is performed using manual methods. The culture pathway adheres to national guidance and is a fully UKAS-accredited method.
Manual testing allows a larger amount of urine to be tested than previous automated method, which enables the laboratory to detect lower bacterial counts (as low as 103 cfu/mL) and also facilitates the follow up of significant organisms grown from mixed cultures.
If the culture result is indicative of urinary tract infection, antibiotic susceptibilities will be tested from the culture growth and will be available 24 hours after the culture result. ‘Direct sensitivities’ are no longer performed. Direct susceptibility testing is not inoculum-controlled, produces inaccurate results and is not UKAS-accredited.
Culture results should be interpreted alongside the microscopy WBC count and clinical signs and symptoms. Significant growth on culture in the absence of pyuria may be suggestive of contamination with regional flora rather than true infection. It should be noted, however, that WBC degrade in urine quite rapidly and delays between sample collection and microscopy may lead to falsely low WBC readings which may account for these findings.
What does the result ‘No significant growth’ mean?
The amount of growth falls below the threshold for urinary tract infection (< 103 cfu/mL).
There is no laboratory evidence of urinary tract infection.
Occasionally, this may be seen in very early stages of infection or in a partially treated urinary tract infection.
Therefore, please send a repeat specimen if symptoms persist.
What does the result ‘mixed growth doubtful significance’ mean?
This means that the culture revealed a heavy growth of at least 3 organisms with no predominating organism; this represents contamination of the urine with the patient’s flora during collection.
This result does not exclude urinary tract infection but it is not possible to determine the causative organism among the mixture of organisms.
If symptoms persist, please send a repeat urine specimen and ensure that patient understands optimal collection technique.
If you are receiving a lot of ‘mixed growth of doubtful significance’ results, please consider the following:
The instructions that patients are given to collect their urine sample
Delays between sample collection and laboratory processing
Poor collection technique is the most common reason for a heavily mixed growth in a urine sample. It is almost impossible to collect a urine sample without any contamination from the normal bacterial flora which inhabits the area surrounding the urethral opening, but optimal collection technique will minimise this contamination and allow the true infective cause to stand out and be identified (a patient instruction leaflet is available).
The time between sample collection and laboratory processing can allow small amounts of contaminating bacterial flora to multiply up to higher amounts prior to laboratory testing, which can result in heavy mixed growth of bacteria on culture. Using a red topped specimen pot containing boric acid preservative will minimise this.
Red topped boric acid containers
The preservative reduces the overgrowth of organisms and, to a lesser extent, reduces the degradation of white cells during transit leading to a more accurate laboratory result for both microscopy and culture.
Red topped boric acid containers are for requests for urine microscopy and culture (MC&S) ONLY. Boric acid container should NOT be used for:
Other urine microbiology tests (e.g. investigations for Chlamydia, Mycobacterium, Schistosomiasis, urinary antigen testing)
Urine samples being analysed by PCR methodology
Urine samples for non-microbiology tests (e.g. biochemistry, virology, pregnancy testing)
Very small urine volumes (<20ml) e.g. neonates
Use of urinary dipsticks: boric acid may inhibit leukocyte esterase dipstick readings; dipstick testing performed on a sample in a boric acid container should be interpreted with caution.
If additional tests are required in addition to urine microscopy and culture, an additional sample in a white-topped universal container should be sent. In this case, it is advised that the mid-stream clean catch urine is collected in a sterile bowl and then transferred to the necessary specimen containers.
If, despite these measures, a patient has recurrent mixed growth reports from multiple urines, it may suggest that your patient has abnormal urinary tract architecture, immunosuppression or other non-infective cause that requires different laboratory investigations or referral to a specialist. If further information is required, please telephone the laboratory and ask to discuss the case with one of our consultant Microbiologists.